The Connecticut Science Center will host its next MakeFest on Saturday, September 18. In celebration of our state’s history of innovation and ingenuity, we will feature local makers, artisans, and tech whizzes to inspire visitors to build and create with their own hands. This time around, the Amistad will dock for its month-long stay in Hartford, so in the spirit of nautical science, we’re turning to shipbuilding for this month’s STEM Career Connections story.
If you have ever been to a beach before, you have most likely seen a docked boat. Maybe you wondered how a ship floats but a pebble sinks. Because you are asking these questions, you are thinking like a scientist! To me, the most surprising part about this question is that boats can weigh tens of thousands of pounds and still stay afloat, but a pebble weighing less than 1 pound will sink. So let us think like engineers and physicists, dive in, and figure out why boats float and how real STEM professionals use this knowledge in everyday life.
Both objects are both made of matter, which makes up everything you touch. When something contains matter, it also has mass, which measures the amount of matter making up an object. It is tempting to say that pounds are a unit of mass, but that is a widespread misconception. The American unit for mass is a slug, and a pound measures the amount of force mass creates in one direction.
Direction is the critical distinction between slugs and pounds. When we say that something weighs 100 pounds, we scientifically say that it produces 100 pounds of force downwards. This downward force exists because of gravity, which, as we know, is not the same everywhere. For example, picture an astronaut who weighs 200 pounds on earth and has a mass of 6.2 slugs. When this astronaut visits the moon, their previous weight of 200 pounds shrinks to 33.2 pounds. However, the astronaut’s mass of 6.2 slugs stays the same because it has nothing to do with the downward directional force of gravity.
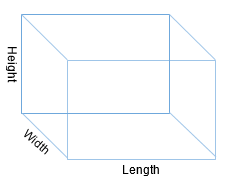 Next, let us think about volume or the amount of 3D space an object fills. For example, imagine you need to put a cardboard box on a shelf. To ensure it fits, you need to know how much space the shelf has and how much space the box takes up. However, if we only measure the length of the box or shelf, we do not have enough information because both objects also have height and width. Therefore, we must calculate the width, height, and length of the shelf to box to see if it will indeed fit. Then, we can multiply all three numbers together and get a new number—volume. If we use “feet,” we get a new unit called a cubic foot (feet x feet x feet).
Next, let us think about volume or the amount of 3D space an object fills. For example, imagine you need to put a cardboard box on a shelf. To ensure it fits, you need to know how much space the shelf has and how much space the box takes up. However, if we only measure the length of the box or shelf, we do not have enough information because both objects also have height and width. Therefore, we must calculate the width, height, and length of the shelf to box to see if it will indeed fit. Then, we can multiply all three numbers together and get a new number—volume. If we use “feet,” we get a new unit called a cubic foot (feet x feet x feet).
Now that we understand mass and volume, we can start to dive into another scientific value called density. We divide mass by volume to get density, so we measure density in units of mass/volume or slugs per foot cubed. Let us investigate a little further by making some real-life comparisons.
When we compare two objects’ densities, the mass of the objects will usually be different, but the amount of space we are examining will always be the same. For example, to compare the densities of blocks of iron and steel, we need to look at amounts that occupy the same space. In other words, we only care about the amount of mass in the same amount of space in both objects. With that, we can now answer the original question!
Pebbles feel heavy compared to their tiny size because they are incredibly dense, or in other words, they have a lot of mass for every unit of space. Of course, an entire boat is much heavier than a pebble, but if we look at a segment of a boat that is the size of a pebble, we will find that the pebble has more mass and still feels heavier. In any fluid, whether it be water, air, or any other gas or liquid, an object will sink if its density is larger than the density of the fluid. Therefore, boats float because they are less dense than water, and pebbles sink because they are denser than water!
So now that we understand how a boat floats, what can we do with this knowledge? First, marine engineers must be aware of volume and mass to keep their boat designs afloat. Suppose they want to use a material that is heavier and denser. In that case, they have to make the boat larger, or increase its volume, to spread out the mass and ensure that the boat’s overall density is less than the water’s density. Conversely, if the company wants to use lighter materials, they can afford to make the vessel smaller or take up less volume.
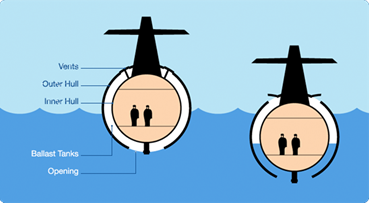
Density is not just helpful in floating; it also makes it possible to sink things purposely! For example, it is beneficial for submarines to drop far below surface level by increasing their density to be greater than water. Submarine engineers do this by using something called a ballast tank. When the captain wants to go deeper in the water, they open the ballast tanks, and incoming water increases the submarine’s mass and density. When its density surpasses that of water, it sinks. The sub can then empty these tanks when the captain wants to float back to the surface.
 Let me introduce an extraordinary physicist named Toby Bothel, who uses mass, volume, and density every day at work. For 34 years, Bothel designed fluid flow engineering for shipbuilding. She needed to know the boat’s density and the density of the water it would traverse. As wild as it sounds, fresh water and saltwater have different densities! You can apply what we’ve learned today to future careers in aerospace (how do planes stay afloat in the air?), submarine-building (how do subs float?), and shipbuilding!
Let me introduce an extraordinary physicist named Toby Bothel, who uses mass, volume, and density every day at work. For 34 years, Bothel designed fluid flow engineering for shipbuilding. She needed to know the boat’s density and the density of the water it would traverse. As wild as it sounds, fresh water and saltwater have different densities! You can apply what we’ve learned today to future careers in aerospace (how do planes stay afloat in the air?), submarine-building (how do subs float?), and shipbuilding!

Nathan Green is a summer intern for the Programs Team at the Connecticut Science Center. He is currently studying Biomedical Engineering at the University of Hartford. He one day hopes to contribute to NASA’s mission to colonize Mars with the help of biotechnology or to advance research in tissue engineering. Prior to joining the Science Center, Nathan worked in childcare.



