Materials to Collect
- Baking soda
- Vinegar
- Plastic bags
- Film canister
Try it Out
- Put some baking soda into a plastic bag. There is no set amount, but we find that about 1 tablespoon works well in a sandwich sized bag.
- Put some vinegar into the plastic bag and QUICKLY seal the bag.
- Watch your bag!
- Now put some baking soda into your film canister.
- Pour some vinegar into the film canister and QUICKLY put the cap on.
- Take a giant step back and watch your canister!
What is the Science?
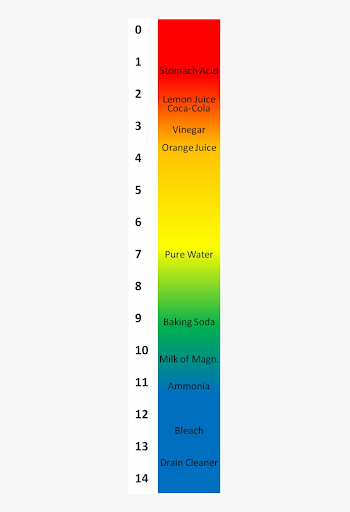
Vinegar is an acid and baking soda is a base. You can find them on different sides of our pH scale. Since they are on opposite sides of the pH scale, when they come in contact with each other – they react! In this reaction, baking soda and vinegar react with each other and form carbon dioxide gas. This gas (the same thing we breathe out) is what causes your plastic bag to puff up and your film canisters to explode! Our plastic bag is flexible so it can expand, but our film canister is rigid and cannot. When the pressure of the carbon dioxide gas builds up too much, it forces the cap off the film canister and sends it sky high!
Ask Your Young Scientists
- What do you notice when we seal our plastic bag? What about when we put the cap on our film canister?
- Why did the plastic bag and the film canister act differently, even though we put the same things inside?
- What might happen if we add more vinegar and baking soda next time? What about less?
More to Explore
- Try varying the amounts of baking soda and vinegar in your plastic bag. What happens if you add more or less?
- Can you find other acidic food items in your home? (Hint: think about what you noticed when vinegar, an acid, came in contact with baking soda. Other acids will react similarly). Safety Note: Please do not use bleach, ammonia, or other household cleaners because they can combine in toxic ways. Mixing different food items is safer.
- Can you create a way to test if materials are acids or bases?
We want to see what you try at home. Share your creation with us on social media by using the #ScienceAtPlay and tagging @CTScienceCenter.

Nick Villagra is a STEM Educator at the Connecticut Science Center, responsible for developing and delivering science experiences, including classroom lab programs, stage shows, and vacation camps. Nick holds a Bachelor’s of Science in Engineering from Swarthmore College. and has been a speaker at the New England Museum Association conference. Always looking to put a unique stamp on the Science Center’s offerings, Nick enjoys incorporating custom-designed 3D printed materials for students to interact with.