Seeking to understand nature’s mysteries is like solving a jigsaw puzzle…except we often don’t have all the pieces. Sometimes there are substances at work that are too small or too hidden for us to be aware of, but even without all the pieces, scientists can think of powerful ideas that help us see the big picture.
19th century Russian scientist Dmitri Mendeleev came up with a powerful idea to help us understand the question: how do different types of matter relate to each other? At the time, people knew that matter could be broken down into elements that behaved in different ways, but it wasn’t clear if there were any patterns governing their behavior.
To investigate this, Mendeleev listed the sixty-three known elements in order of their weight and saw something exciting—there was a regularity, or “periodicity,” to the elements’ characteristics. If elements were eight places from each other on the list, they had the same chemical properties. For instance, elements 3 (lithium), 11 (sodium), 19 (potassium), etc. had the same property of reacting intensely with water. Elements 4 (beryllium), 12 (magnesium), 20 (calcium) had their own set of similar properties, and so forth. He arranged these groups into a “periodic table of elements.” Nature was starting to look pretty orderly.
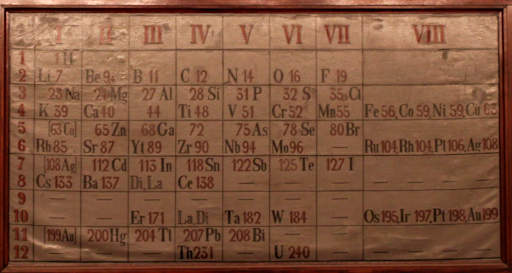
The original Periodic Table of Elements. Source: Wikimedia
However, there was one snag. Certain sections of the list didn’t follow the pattern; the only way to make the table work was to leave in some odd gaps. Mendeleev didn’t have all the pieces, but it didn’t matter. He had the big picture. He predicted that elements existed like eka-aluminum that would fill the gaps; he figured they just hadn’t been found yet. Sure enough, less than ten years after Mendeleev first presented his table, the element gallium was discovered that neatly matched the properties of his theoretical eka-aluminum.
Not every scientist’s idea ends up being correct, though. Two centuries before Mendeleev, scientists were investigating the question: what happens when things burn? They saw that flames emanate from burning objects, so they reasoned that a yet-to-be-discovered substance they called phlogiston would radiate away from objects as they burned. Here’s how it was supposed to work: an object like wood contained a certain amount of phlogiston. When it burned, the phlogiston was escaping from it. The more it burned, the more phlogiston escaped, and the lighter the object would get. The idea was logical, intuitive, and… wrong. Experiments done in the 18th century showed that substances actually get heavier as they burn, but many scientists were still convinced that phlogiston had to exist to explain burning; German scientist Georg Stahl went so far as to suggest the bizarre idea that phlogiston must somehow have a negative mass.
French scientist Antoine Lavoisier countered with an alternative explanation for how burning works that dispensed with phlogiston. His new theory turned the old one on its head; instead of thinking of burning as a substance escaping an object into the air, he saw it as a substance in the air reacting with the object. He called that substance oxygen.
The mysterious weight increase of a burning object now made sense—oxygen was making it heavier. From then on, the scientific community accepted that phlogiston was about as real as the tooth fairy.
To this day, scientists continue to use their reasoning and imagination to help explain puzzling phenomena. Imagination is what gives science direction—discoveries aren’t made very often by just blindly searching; it’s important to know what we’re looking for, and how it fits into the big picture. Sometimes, finding proof of these hypothetical things can seem impossible. In 1916, Albert Einstein predicted the existence of gravitational waves as part of his general theory of relativity, but doubted that we’d ever have instruments sensitive enough that could prove it definitively. Never ones to back down from a challenge, physicists built the $395 million LIGO (Laser Interferometer Gravitational-Wave Observatory) that made history in 2015 when it detected gravitational waves for the first time. Einstein’s theory was spot on, but it took 100 years for experimentalists to catch up to his brilliant mind.

LIGO facility in Livingston, Louisiana, consisting of two 2.5 mile arms arranged in an L shape. Lasers shine through vacuum tubes housed in each arm. Source: Wikipedia
Our senses are limited, and modern technology can only expand our senses so far—but there is no limit to our imagination. As long as scientists continue to envision things beyond what we can currently perceive, we’ll continue to unravel the deepest mysteries of nature.
Stay connected! Be sure to subscribe to Down to a Science— The Official Blog of the Connecticut Science Center and follow us on social media.

Nick Villagra is a STEM Educator at the Connecticut Science Center, responsible for developing and delivering science experiences, including classroom lab programs, stage shows, and vacation camps. Nick holds a Bachelor’s of Science in Engineering from Swarthmore College. and has been a speaker at the New England Museum Association conference. Always looking to put a unique stamp on the Science Center’s offerings, Nick enjoys incorporating custom-designed 3D printed materials for students to interact with.


